Efficacy data in HCC CABOMETYX® (cabozantinib) monotherapy
On This Page:
CELESTIAL: largest phase 3 trial of HCC patients previously treated with sorafenib1,2
The CELESTIAL trial was a randomized (2:1), double-blind, phase 3 trial of 707 patients with HCC (Child-Pugh A).1,2
2:1 randomization (N=707)* | |
|---|---|
CABOMETYX | Placebo |
Treatment until disease progression or unacceptable toxicity | |
Primary endpoint | Secondary endpoints |
- *
-
707 patients were randomized at the time of the second interim analysis for OS. In total, CELESTIAL enrolled 773 patients.2
Inclusion criteria2:
- Pathologic diagnosis of HCC not amenable to curative treatment
- Child-Pugh A
- Received prior sorafenib
- Prior treatment with 1-2 systemic therapies, with progression following at least 1 therapy
- ECOG PS of 0 or 1
Prespecified stratification1:
- Disease etiology (HBV with or without HCV, HCV without HBV, other)
- Region (Asia, other)
- Presence of macrovascular invasion and/or extrahepatic spread of disease (yes, no)
Child-Pugh scores were assessed by the investigator at the time of each radiographic disease assessment every 8 weeks.3
In CELESTIAL, 71% of CABOMETYX patients were second-line5
CELESTIAL did not exclude patients who had4,5:
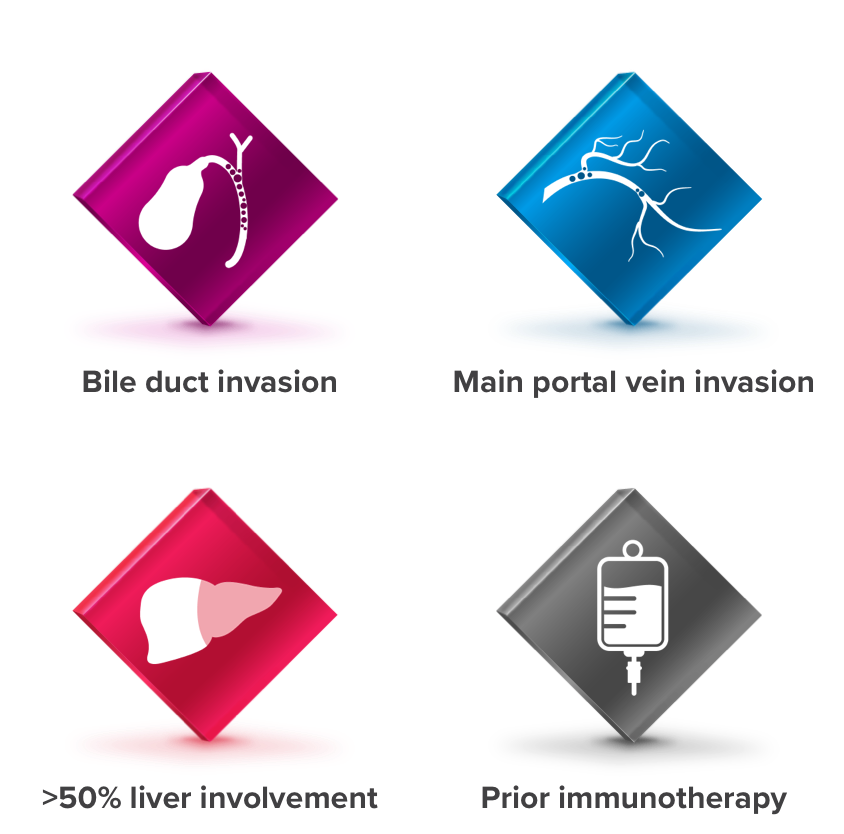
CELESTIAL included a range of patients4
Baseline Patient Characteristics
No. (%) | |||
CABOMETYX | Placebo | ||
Age (years) | |||
Median (range) | 64 (22-86) | 64 (24-86) | |
Sex | |||
Male / female | 379 (81) / 91 (19) | 202 (85) / 35 (15) | |
Geographic region | |||
Asia / Europe | 116 (25) / 231 (49) | 59 (25) / 108 (46) | |
United States and Canada | 108 (23) | 59 (25) | |
Australia and New Zealand | 15 (3) | 11 (5) | |
Race | |||
White / Asian | 264 (56) / 159 (34) | 130 (55) / 82 (35) | |
Black or African American | 8 (2) | 11 (5) | |
Other and not reported | 39 (9) | 14 (6) | |
Number of prior systemic anticancer regimens for HCC | |||
1 | 335 (71) | 174 (73) | |
2 | 130 (28) | 62 (26) | |
≥3 | 2 (<1) | 1 (<1) | |
Prior systemic anticancer therapy | |||
Sorafenib | 470 (100) | 237 (100) | |
Anti-PD-1 or PD-L1 | 14 (3) | 3 (1) | |
Other | 116 (25) | 56 (24) | |
Etiology of disease | |||
HBV | 178 (38) | 89 (38) | |
HCV | 113 (24) | 55 (23) | |
Dual HBV / HCV infection | 8 (2) | 4 (2) | |
Alcohol | 112 (24) | 39 (16) | |
Nonalcoholic steatohepatitis | 43 (9) | 23 (10) | |
Other and unknown | 99 (21) | 63 (27) | |
ECOG PS | |||
0 / 1 / 2 | 245 (52) / 224 (48) / 1 (<1) | 131 (55) / 106 (45) / 0 | |
BCLC stage | |||
B / C | 42 (9) / 427 (91) | 23 (10) / 214 (90) | |
Alpha-fetoprotein | |||
<400 ng/mL | 278 (59) | 136 (57) | |
≥400 ng/mL | 192 (41) | 101 (43) | |
Extrahepatic spread of disease | 369 (79) | 182 (77) | |
Macrovascular invasion | 129 (27) | 81 (34) | |
Extrahepatic spread of disease and/or macrovascular invasion | 398 (85) | 200 (84) | |
Local liver-directed non-radiation anticancer therapy | 209 (44) | 113 (48) | |
Total duration of treatment of prior sorafenib | |||
Median, months | 5.3 | 4.8 | |

Patients who progressed from Child-Pugh A to Child-Pugh B within the first 8 weeks of treatment remained in the trial until disease progression or unacceptable toxicity (73/707)3

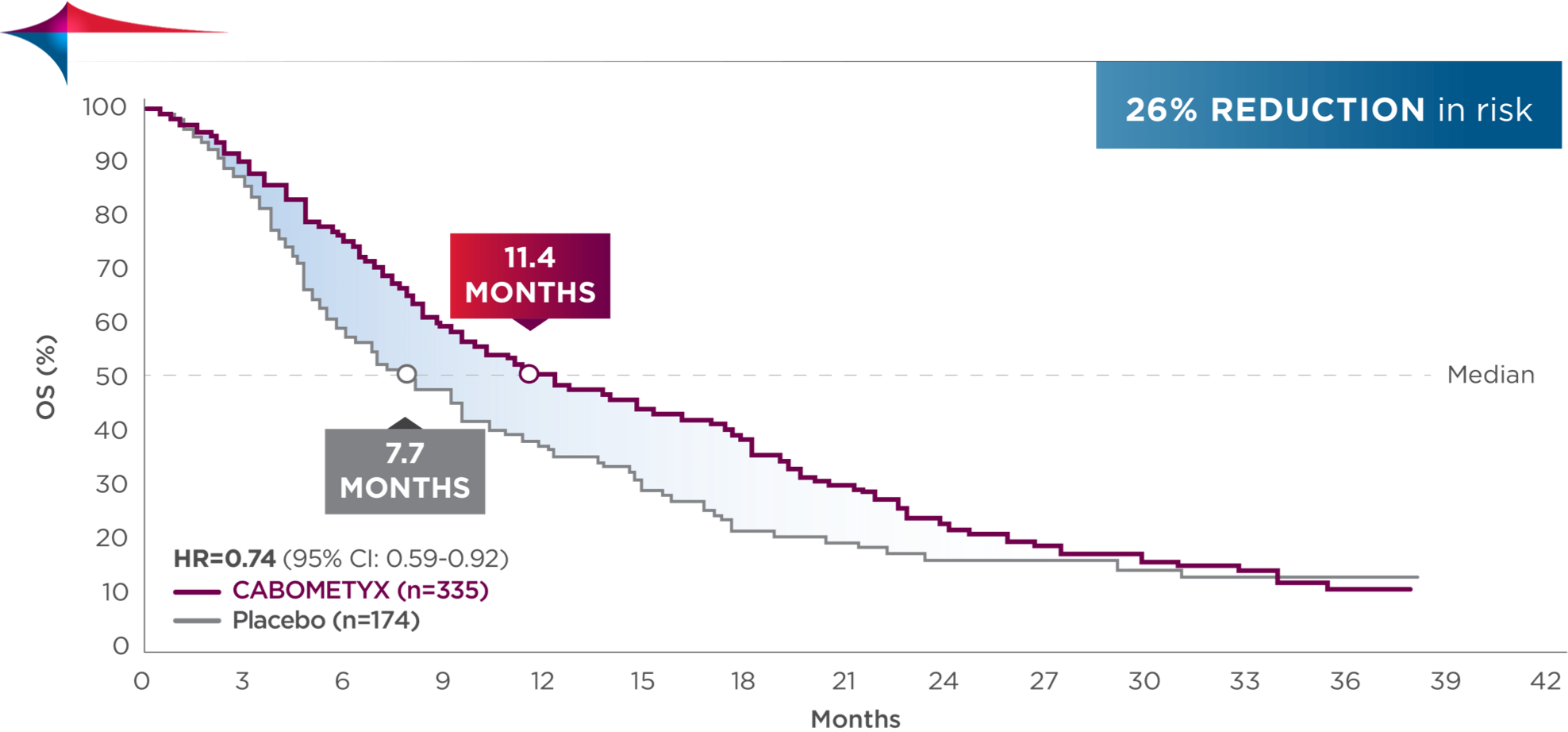
OS results
Superior OS in the treatment of 2L HCC1
Primary endpoint: OS (ITT population who received at least one prior therapy, including sorafenib; median)
| 10.2 | MONTHS CABOMETYX (n=470) |
8.0 | MONTHS Placebo (n=237) |
(HR=0.76; 95% CI: 0.63-0.92; P=0.0049) |
In a prespecified exploratory subgroup analysis of patients who received only 1 prior systemic therapy
CABOMETYX exceeded 11 months median OS (second-line)2
Subgroup analysis: OS5†
†No statistical procedure was employed for controlling type 1 error. Results should be considered hypothesis generating.
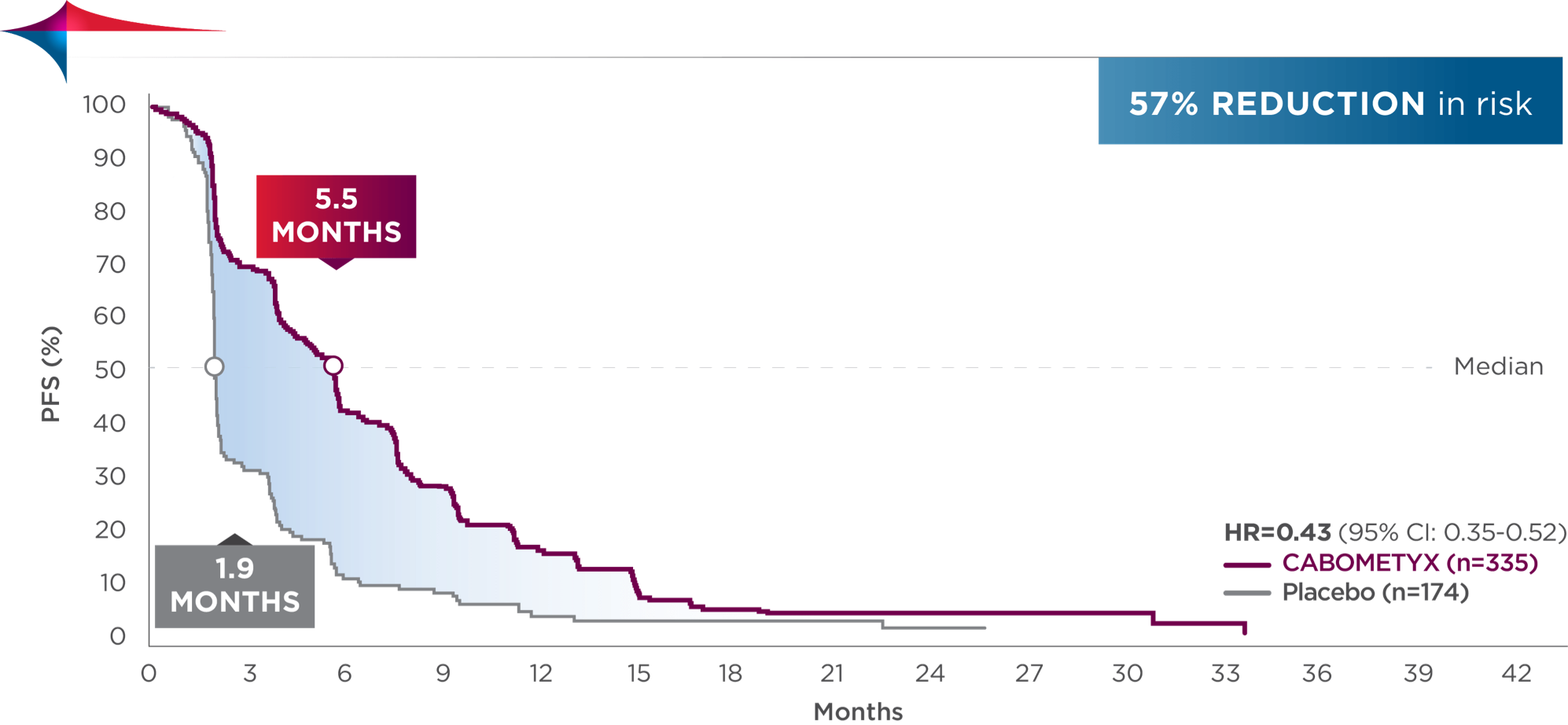
PFS results
Superior PFS in the treatment of 2L HCC1
Secondary endpoint: PFS (ITT population who received at least one prior therapy, including sorafenib; median)
5.2 | MONTHS | 1.9 | MONTHS | (HR=0.44; 95% CI: 0.36-0.52; P<0.0001) |
In a prespecified exploratory subgroup analysis of patients who received only 1 prior systemic therapy
CABOMETYX exceeded 5 months median PFS (second-line)2
Subgroup analysis: PFS5‡
‡No statistical procedure was employed for controlling type 1 error. Results should be consider hypothesis generating.
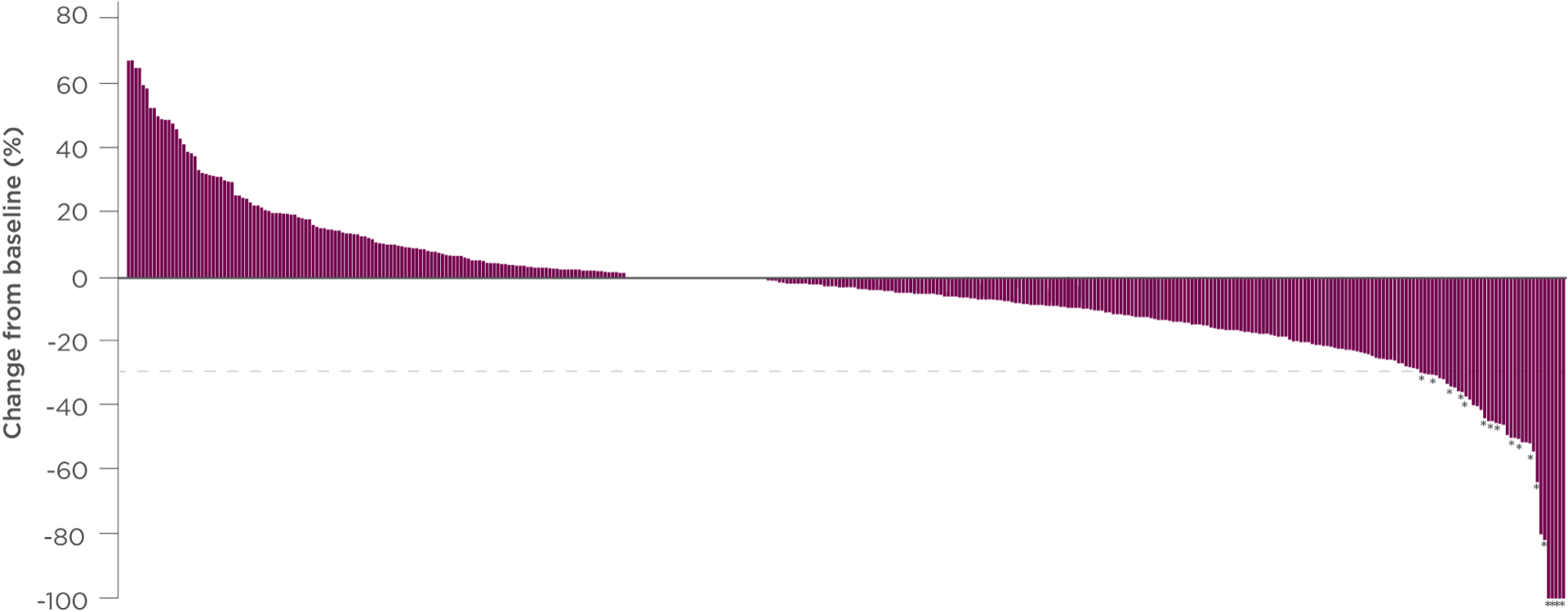
CABOMETYX improved tumor control for patients with HCC1,4-7
With CABOMETYX, 47% of patients experienced tumor shrinkage in the ITT analysis6§
Reduction in target lesion from baseline
Inclusion criteria6
-
Each vertical line corresponds to one patient. The plot represents the best percentage change in tumor size from baseline in the evaluable patient population, as determined by the investigators, per RECIST v1.1
-
Among patients with baseline and postbaseline target-lesion assessment, 222 of 388 patients in the CABOMETYX arm and 27 of 205 patients in the placebo arm had a decrease from baseline
Some patients are not represented due to lack of evaluable postbaseline assessment, censoring (per PFS rules) before first evaluable postbaseline assessment, lack of target lesions, and/or incomplete or inevaluable target-lesion assessment. Data from time points after the first date of any of the censoring events defined for the primary PFS analysis were excluded.5
§Confirmed partial responses.5
Tumor response observed in the ITT population in the CELESTIAL trial1,5
ORR was 4% among patients receiving CABOMETYX.|| ORR was defined as a ≥30% reduction in target tumor size from baseline, per RECIST v1.1.1,7
Secondary Endpoint: ORR (ITT)1,2,4
No. (%) | ||
|---|---|---|
CABOMETYX | Placebo | |
Confirmed ORR|| [95% CI] | 18 (4) | 1 (0.4) |
P=0.0086 | ||
Stable disease | 282 (60) | 78 (33) |
Progressive disease | 98 (21) | 131 (55) |
Not evaluable or missing | 72 (15) | 27 (11) |
||All responses were confirmed partial responses, as assessed by the investigators per RECIST v1.1.4
- Disease control rate was defined as the percentage of patients with a complete response, partial response, or stable disease, as measured by RECIST v1.1. Stable disease may reflect the natural history of disease rather than any effect of the drug
With CABOMETYX, 64% disease control rate was observed4

National Comprehensive Cancer Network® (NCCN®)
Cabozantinib (CABOMETYX) is recommended as a Category 1 subsequent-line treatment option for HCC¶
-
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate. - ¶
-
For certain patients with Child-Pugh Class A liver function only, following disease progression on first-line systemic treatment. Data reflect use after sorafenib.
2L=second-line; BCLC=Barcelona Clinic Liver Cancer; CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; HBV=hepatitis B virus; HCC=hepatocellular carcinoma; HCV=hepatitis C virus; HR=hazard ratio; ITT=intent to treat; ORR=objective response rate; OS=overall survival; PD-1=programmed cell death protein-1; PD-L1/L2=programmed death ligand-1/2; PFS=progression-free survival; QD=once daily: RECIST=Response Evaluation Criteria in Solid Tumors.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54-63.
- El-Khoueiry AB, Meyer T, Cheng AL, et al. Safety and efficacy of cabozantinib for patients with advanced hepatocellular carcinoma who advanced to Child–Pugh B liver function at study week 8: a retrospective analysis of the CELESTIAL randomized controlled trial. BMC Cancer. 2022;22(1):377.
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma [supplementary appendix]. N Engl J Med. 2018;379(1):54-63.
- Data on file. Exelixis, Inc.
- Merle P, Rimassa L, Ryoo B-Y, et al. Assessment of tumor response, AFP response, and time to progression in the phase 3 CELESTIAL trial of cabozantinib versus placebo in advanced hepatocellular carcinoma. Poster presented at: ESMO 20th World Congress on Gastrointestinal Cancer; June 20-23, 2018; Barcelona, Spain.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hepatobiliary Cancers V.1.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed April 4, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org.

