Dosing in CABOMETYX® (cabozantinib) HCC monotherapy
On This Page:
Available in 3 strengths to help you find the right dose for your patients when needed1
| RECOMMENDED STARTING DOSE* |
FIRST REDUCTION |
SECOND REDUCTION |
|---|---|---|
60 mg once daily |
40 mg once daily |
20 mg once daily† |
| RECOMMENDED STARTING DOSE* | |
|---|---|
| FIRST REDUCTION | |
| SECOND REDUCTION |
Tablets shown are not actual size.
*Until disease progression or unacceptable toxicity administered as recommended.1
†If previously receiving lowest dose, resume at same dose. If lowest dose not tolerated, discontinue CABOMETYX.1
In the CELESTIAL trial, the mean average daily dose was 37 mg2
Reduce dose of CABOMETYX for patients with hepatic impairment1
Child-Pugh B: Reduce the starting dose of CABOMETYX to 40 mg once daily in patients with moderate hepatic impairment.
- Avoid CABOMETYX in patients with severe hepatic impairment (Child-Pugh C)
How to administer CABOMETYX1

DO NOT ADMINISTER CABOMETYX WITH FOOD
Administer CABOMETYX at least 1 hour before or at least 2 hours after eating

Swallow whole
DO NOT CRUSH TABLET
-
Withhold CABOMETYX for at least 3 weeks prior to elective surgery, including dental surgery. Do not administer CABOMETYX for at least 2 weeks after major surgery and until adequate wound healing is observed1
-
Do not substitute CABOMETYX tablets with cabozantinib capsules1
-
Do not take a missed dose within 12 hours of the next dose1
-
Modify the dose for certain patients with hepatic impairment and for patients taking drugs known to strongly induce or inhibit CYP3A41
Pharmacokinetics
The predicted terminal half-life of CABOMETYX is approximately 99 hours.1
Dose modifications1
You may need to adjust the CABOMETYX dose based on individual patient safety and tolerability. If ARs occur, consider supportive care and/or adjust the dose.
For intolerable Grade 2 ARs, Grade 3-4 ARs, and ONJ
Withhold CABOMETYX
Wait until improvement or resolution (return to baseline or resolution to Grade 1)
Reduce the dose by 20 mg
If previously receiving 20 mg once daily, resume at same dose.
If not tolerated, discontinue CABOMETYX
Permanently discontinue CABOMETYX for Grade 3 or 4 hemorrhage, development of a GI perforation or Grade 4 fistula, acute myocardial infarction or Grade 2 or higher cerebral infarction, Grade 3 or 4 arterial thromboembolic events or Grade 4 venous thromboembolic events, Grade 4 hypertension/hypertensive crisis or Grade 3 hypertension/hypertensive crisis that cannot be controlled, nephrotic syndrome, or reversible posterior leukoencephalopathy syndrome.1
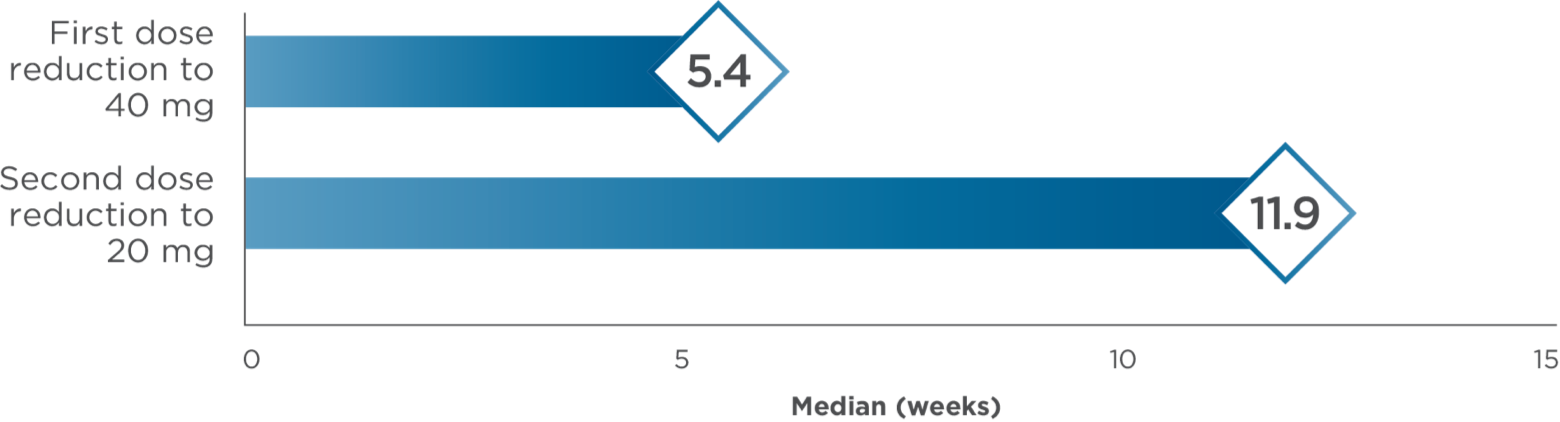
Median time to dose reductions3,4
When strong CYP3A4 inhibitors cannot be avoided1

Reduce the daily dose of CABOMETYX if concomitant use with strong CYP3A4 inhibitors cannot be avoided.
Resume CABOMETYX at the dose used prior to initiating the strong CYP3A4 inhibitor 2-3 days after discontinuation of the strong inhibitor.
Examples of strong CYP3A4 inhibitors‡:
- Boceprevir
- Clarithromycin
- Conivaptan
- Grapefruit juice
- Indinavir/ritonavir
- Itraconazole
- Ketoconazole
- Lopinavir/ritonavir
- Nefazodone
- Nelfinavir
- Posaconazole
- Ritonavir
- Saquinavir/ritonavir
- Voriconazole
When strong CYP3A4 inducers cannot be avoided1

Increase the daily dose of CABOMETYX if concomitant use with strong CYP3A4 inducers cannot be avoided.
Resume CABOMETYX at the dose used prior to initiating the strong CYP3A4 inducer 2-3 days after discontinuation of the strong inducer.
- Do not exceed a daily dose of 80 mg
Examples of strong CYP3A4 inducers‡:
- Rifampin
- Phenytoin
- Carbamazepine
- St. John’s wort
‡Examples listed may not be comprehensive.

For eligible patients who have been prescribed CABOMETYX: Dose Exchange Program
Provides a free 15-tablet supply in the lower dose to help patients who require a dose reduction§||
- §
-
Additional restrictions and eligibility rules apply.
- ||
-
Patients are required to return any unused product.
This description of the Exelixis Access Services® (EASE) program is for informational purposes only. Exelixis® makes no representation or guarantee concerning reimbursement or coverage for any service or item. Information provided through the Exelixis Access Services program does not constitute medical or legal advice and is not intended to be a substitute for a consultation with a licensed health care provider, legal counsel, or applicable third-party payer(s). Exelixis reserves the right to modify the program at any time without notice.
AR=adverse reaction; CYP3A4=cytochrome P450 family 3 subfamily A member 4; GI=gastrointestinal; HCC=hepatocellular carcinoma; ONJ=osteonecrosis of the jaw.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Data on file. Exelixis, Inc.
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Assessment report: CABOMETYX. September 2018. Accessed 2019.
- Schwartz G, Darling JO, Mindo M, Damicis L. Management of adverse events associated with cabozantinib treatment in patients with advanced hepatocellular carcinoma. Target Oncol. 2020;15(4):549-565. doi:10.1007/s11523-020-00736-8


