Efficacy data in 2L DTC CABOMETYX® (cabozantinib) monotherapy
In patients who are RAI-R or ineligible and progressed on a prior VEGFR-targeted therapy1
On This Page:
Meaningful results led to early unblinding in the COSMIC-311 trial2
COSMIC-311 was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial.1,3
2:1 randomization | ||||
|---|---|---|---|---|
| ||||
Treatment until disease progression | ||||
UNBLINDED TRIAL‡ | Cabozantinib 60 mg | |||
Post-treatment follow-up§ | ||||
The multiple primary efficacy outcome measures assessed ORR in the first 100 patients (OITT) after 6 months of enrollment and PFS in all patients randomly assigned (ITT). The study was designed such that it would be considered positive if either of the primary endpoints were met. Median follow-up was 6.2 months (IQR: 3.4–9.2) for the ITT population and 8.9 months (IQR: 7.1–10.5) for the OITT population. Median duration of treatment exposure in the safety population was 4.4 months (IQR: 2.1-7.3) for the CABOMETYX patients and 2.3 months (IQR: 1.6-5.6) for the placebo group. An updated analysis, with median follow-up of 10.1 months, evaluated a total of 258 randomized patients.1,3,4
- *
-
BSC included dose modification and dose interruption. After unblinding, BSC included potentially receiving open-label cabozantinib.1,3
- †
-
Tumor response and progression were assessed by MRI or CT. Tumor assessments were performed every 8 weeks after randomization for 12 months, then every 12 weeks thereafter.3
- ‡
-
An IDMC for COSMIC-311 recommended ending enrollment and unblinding sites and patients based on cabozantinib reducing the risk of disease progression or death by 78% (HR=0.22; 95% CI: 0.14-0.35; P<0.0001).2
Multiple primary efficacy outcome measures1
-
PFS
- ORR
Stratification factors1
-
Age ≤65 years
-
Prior lenvatinib
Baseline characteristics in ITT population1,3
| Total Population | ||
| Median age, years (range) ECOG 0 ECOG 1 |
65 (31-85) 46% 54% |
|
| CABOMETYX | Placebo | |
| One previous VEGFR TKI Two previous VEGFR TKIs |
73% (91/125) 27% (34/125) |
77% (48/62) 23% (14/62) |
73% of patients received CABOMETYX after one previous systemic therapy3§
- §
-
Updated analysis (N=258).1

CABOMETYX delivered a significant benefit in the primary PFS analysis1
Median PFS was not reached in the primary analysis (n=125, 95% CI: 5.7-NE) vs PFS of 1.9 months with placebo (n=62, 95% CI: 1.8-3.6); HR=0.22 (95% CI: 0.14-0.35); P<0.0001.
Early and sustained separation demonstrated at updated analysis–with a median PFS of 11 months1
Updated analysis
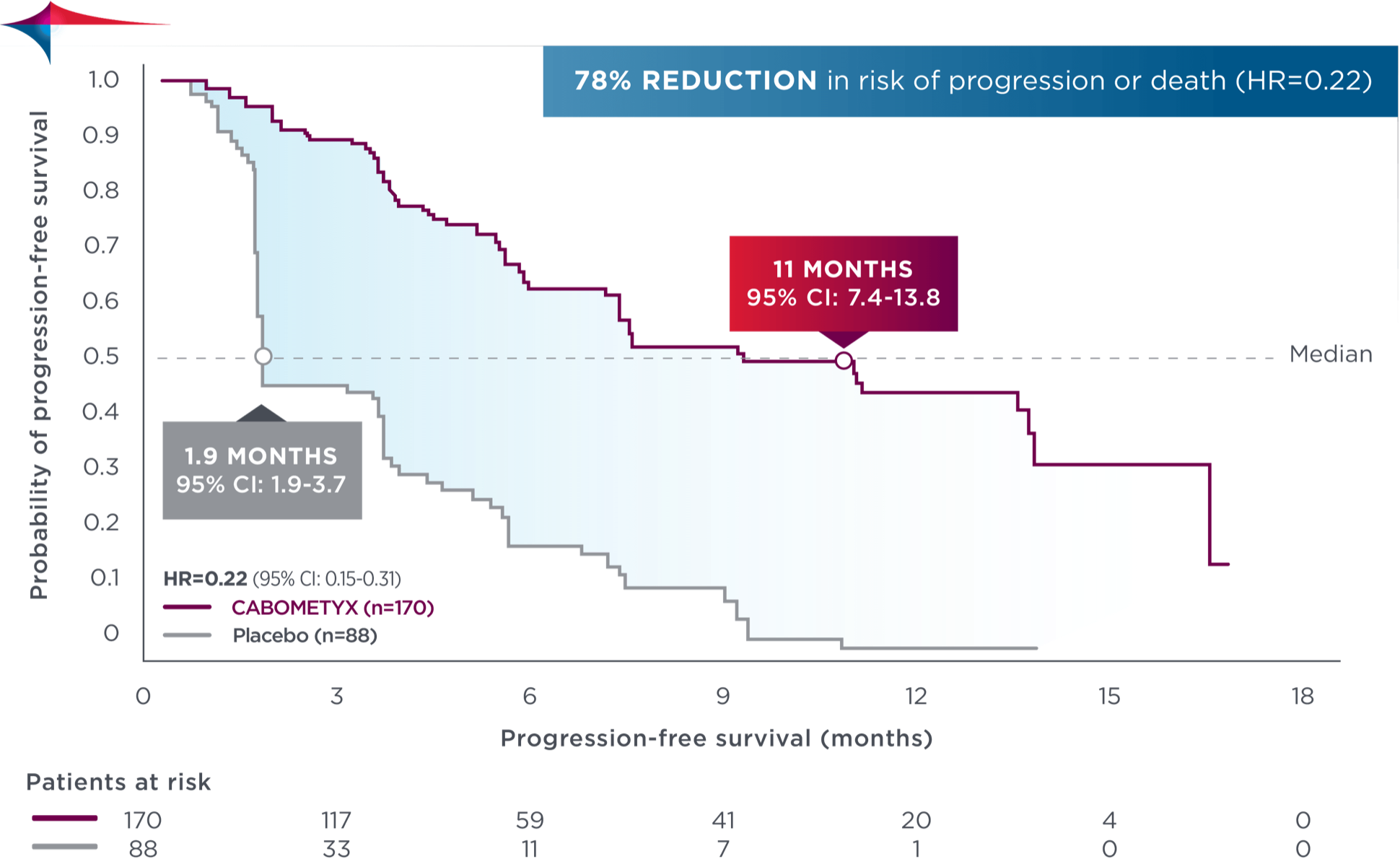
No formal statistical testing was conducted at the time of the updated analysis.
78% reduction in risk of progression or death in both primary and updated analyses
Tumor response observed at the OITT analysis3
In the COSMIC-311 trial, the overall response rate (ORR) did not reach a prespecified endpoint for statistical significance (critical P value=0.01). Data below cover tumor response information collected, inclusive of ORR.1,3
| CABOMETYX | Placebo | |
| ORR, % (95% CI)‖ Stable disease, % (n/N)¶ Disease control rate, % (n/N)** |
15 (7-26) 69 (46/67) 84 (56/67) |
0 (0-11) 42 (14/33) 42 (14/33) |
- ‖
-
P=0.0281; all responses confirmed were partial responses.3
- ¶
-
Stable disease is defined as neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for PD.5
Stable disease may reflect the natural history of disease rather than any effect of the drug. - **
-
Disease control rate was defined as the percentage of patients with a complete response, partial response, or stable disease, as measured by RECIST 1.1.3
76% of patients experienced tumor shrinkage with CABOMETYX at the OITT analysis3††
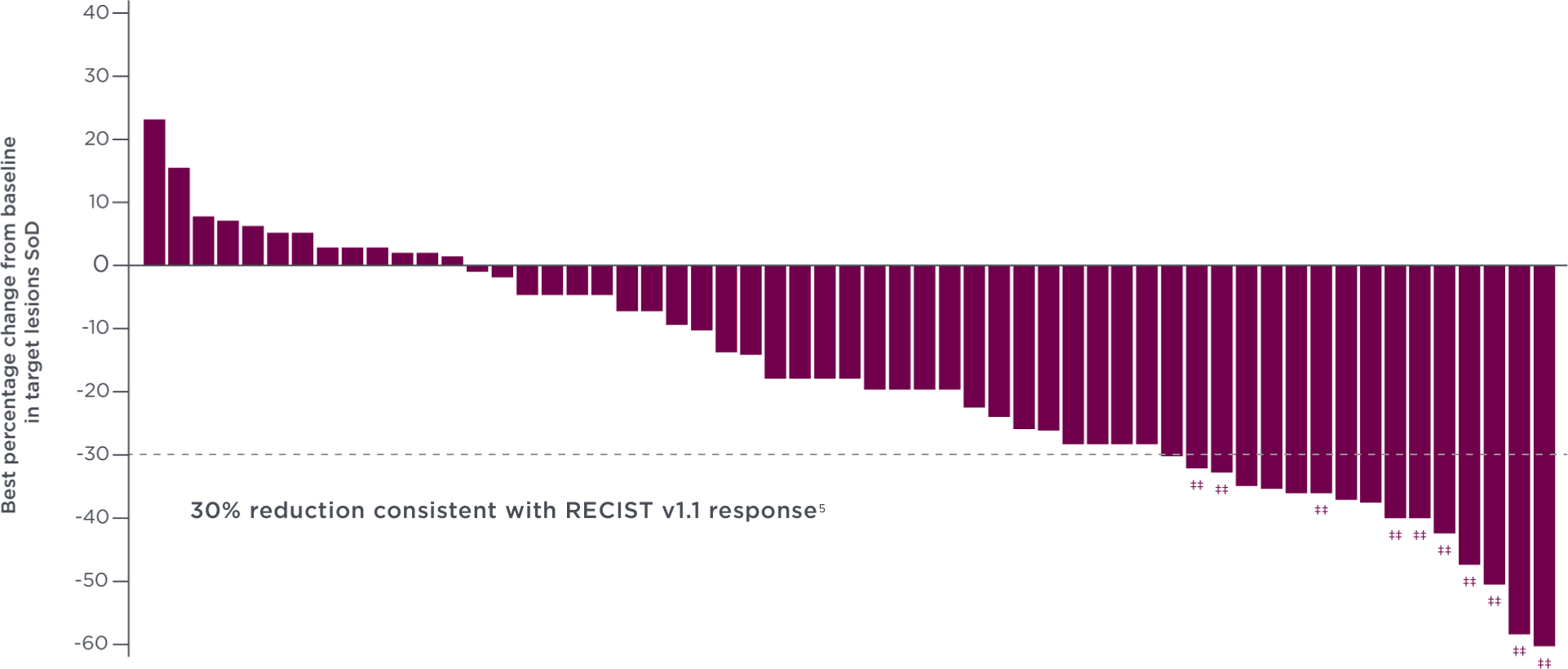
-
Each vertical line represents 1 patient. The plot represents the best percentage change in tumor size from baseline in the evaluable patient population, as determined by the investigators, per RECIST 1.1.3††
- Among patients with baseline and postbaseline target lesion assessment, 44 (76%) of 58 patients with ≥1 postbaseline target lesion assessment in the CABOMETYX arm had some level of reduction in target lesions compared with 9 (29%) of 31 patients in the placebo arm3
- ††
-
Data from OITT analysis of evaluable patients.3
- ‡‡
-
Confirmed partial response.3

National Comprehensive Cancer Network® (NCCN®)
Cabozantinib (CABOMETYX) has a Category 1 recommendation for patients with locally recurrent, advanced, and/or metastatic RAI-R papillary thyroid cancer that has progressed following VEGFR-targeted therapy§§
-
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate. - §§
-
If progression after lenvatinib and/or sorafenib.
2L=second-line; BIRC=blinded independent radiology committee; BSC=best supportive care; CI=confidence interval; CT=computed tomography; DTC=differentiated thyroid cancer; ECOG=Eastern Cooperative Oncology Group performance status; HR=hazard ratio; IDMC=independent data-monitoring committee; IQR=interquartile range; ITT=intent to treat; MRI=magnetic resonance imaging; NE=not estimable; OITT=ORR intent to treat; ORR=overall response rate; PD=progressive disease; PFS=progression-free survival; QD=every day; RAI-R=radioactive iodine-refractory; RECIST=Response Evaluation Criteria in Solid Tumors; SoD=sum of diameter; TKI=tyrosine kinase inhibitor; VEGFR=vascular endothelial growth factor receptor.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Exelixis press release. December 21, 2020. https://ir.exelixis.com/news-releases/news-release-details/exelixis-announces-cabozantinib-significantly-improved
- Brose MS, Robinson B, Sherman SI, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIS-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(8):1126-1138. doi:10.1016/S1470-2045(21)00332-6
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Assessment report: CABOMETYX. September 2018. Accessed September 15, 2022.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Cancer V.3.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed November 1, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org.

