CABOMETYX® (cabozantinib) aRCC data in combination with OPDIVO® (nivolumab)
On This Page:
Explore data for the #1 prescribed TKI + IO in 1L aRCC
Based on IQVIA BrandImpact data as of December 2024. Subject to change without notice.1

Related Content
CheckMate-9ER study design and baseline characteristics
Median follow-up was 18.1 months (range: 10.6-30.6 months) with an extended follow-up analysis at 67.6 months (range 60.2-80.2 months).2,3
An open-label trial vs sunitinib in patients with previously untreated aRCC2,4
1:1 randomization2 | ||||
|---|---|---|---|---|
| ||||
Treatment until disease progression or unacceptable toxicity4* | ||||
Primary endpoint4,6 | Secondary endpoints4,6 | Exploratory endpoint2‡ | ||
- Excluded patients with autoimmune disease or other medical conditions requiring systemic immunosuppression4
- Primary analysis included a median follow-up of 18.1 months (range: 10.6-30.6 months)2,3
- Pre-planned final OS analysis was conducted at a median follow-up of 32.9 months (range: 25.4-45.4 months) after observing 271 events4,7,8
- Extended 5-year minimum follow-up analysis was conducted with a median follow-up of 67.6 months (range: 60.2-80.2 months)3
Stratification factors4
- IMDC risk group (favorable vs intermediate vs poor)
- PD-L1 tumor expression (≥1% vs <1% or indeterminate)
- Geographic region (United States, Canada, and Western and Northern Europe vs rest of world)§
The starting dose of CABOMETYX was 40 mg in CheckMate-9ER4
- *
-
Tumor assessments were performed at baseline, after randomization at Week 12, then every 6 weeks until Week 60, and then every 12 weeks thereafter. Treatment beyond RECIST v1.1–defined disease progression was permitted if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. OPDIVO dosing was not to exceed a total of 2 years (from cycle 1).4,9
- †
-
PFS and ORR were assessed by BICR.4
- ‡
-
Other exploratory endpoints included biomarkers, PK, immunogenicity, and PFS-2.2,5
- §
-
Western and Northern European countries included: Czechia, Germany, Greece, Italy, Poland, Romania, Russian Federation, Spain, and the United Kingdom of Great Britain and Northern Ireland.3

CheckMate-9ER included 78% of patients who were intermediate/poor risk2
No. (%) | |||
CABOMETYX + OPDIVO | sunitinib | ||
Age (years)2 |
|
| |
<65 | 191 (59.1) | 210 (64.0) | |
≥65 | 132 (40.9) | 118 (36.0) | |
Geographic region3 |
|
| |
US/Canada/W. Europe/N. Europe‖ | 158 (48.9) | 161 (49.1) | |
Rest of world | 165 (51.1) | 167 (50.9) | |
Karnofsky performance status3 |
|
| |
70 | 14 (4.3) | 18 (5.5) | |
80 | 52 (16.1) | 67 (20.4) | |
90 | 110 (34.1) | 112 (34.1) | |
100 | 147 (45.5) | 129 (39.3) | |
Not reported | 0 | 2 (0.6) | |
Baseline IMDC prognostic score2 |
|
| |
Favorable | 74 (22.9) | 72 (22.0) | |
Only 22% were IMDC favorable risk2 |
|
| |
Intermediate | 188 (58.2) | 188 (57.3) | |
Poor | 61 (18.9) | 68 (20.7) | |
78% were IMDC intermediate/poor risk2 |
|
| |
Time from diagnosis to randomization3 |
|
| |
<1 year | 210 (65.0) | 214 (65.2) | |
≥1 year | 112 (34.7) | 111 (33.8) | |
Not reported | 1 (0.3) | 3 (0.9) | |
Prior nephrectomy2 |
|
| |
Yes | 222 (68.7) | 233 (71.0) | |
No | 101 (31.3) | 95 (29.0) | |
30% had primary tumor intact2 (no prior nephrectomy) |
|
| |
Prior radiotherapy3 |
|
| |
Yes | 46 (14.2) | 45 (13.7) | |
No | 277 (85.8) | 283 (86.3) | |
PD-L1+ status3 |
|
| |
≥1% | 81 (25.1) | 81 (24.7) | |
<1% or indeterminate | 232 (71.8) | 240 (73.2) | |
Not reported | 10 (3.1) | 7 (2.1) | |
Sarcomatoid features3 |
|
| |
Yes | 34 (10.5) | 41 (12.5) | |
No | 279 (86.4) | 278 (84.8) | |
Not reported | 10 (3.1) | 9 (2.7) | |
Stage at initial diagnosis3 |
|
| |
Stage IV | 167 (51.7) | 173 (52.7) | |
Non-stage IV | 150 (46.4) | 148 (45.1) | |
Not reported | 6 (1.9) | 7 (2.1) | |
No. of metastatic sites2¶ |
|
| |
1 | 63 (19.5) | 69 (21.0) | |
≥2 | 259 (80.2) | 256 (78.0) | |
~80% had ≥2 organs with metastases2 |
|
| |
Most common sites of metastasis2 |
|
| |
Lung | 238 (73.7) | 249 (75.9) | |
75% had lung metastases2 |
|
| |
Lymph node | 130 (40.2) | 131 (39.9) | |
Bone | 78 (24.1) | 72 (22.0) | |
23% had bone metastases2 |
|
| |
Liver | 73 (22.6) | 53 (16.2) | |
19% had liver metastases2 |
|
| |
Adrenal gland | 36 (11.1) | 36 (11.0) | |
CheckMate-9ER studied a range of patients, including those with a high burden of disease2
- ‖
-
Western and Northern European countries included: Czechia, Germany, Greece, Italy, Poland, Romania, Russian Federation, Spain, and the United Kingdom of Great Britain and Northern Ireland.3
- ¶
-
Data are for tumor sites defined at baseline by the investigators according to RECIST v1.1. The number of target or nontarget lesions at baseline was not reported for 1 patient in the CABOMETYX + OPDIVO group and for 3 patients in the sunitinib group.2

Progression-free survival (PFS), primary endpoint4,6
Primary analysis in the ITT population4
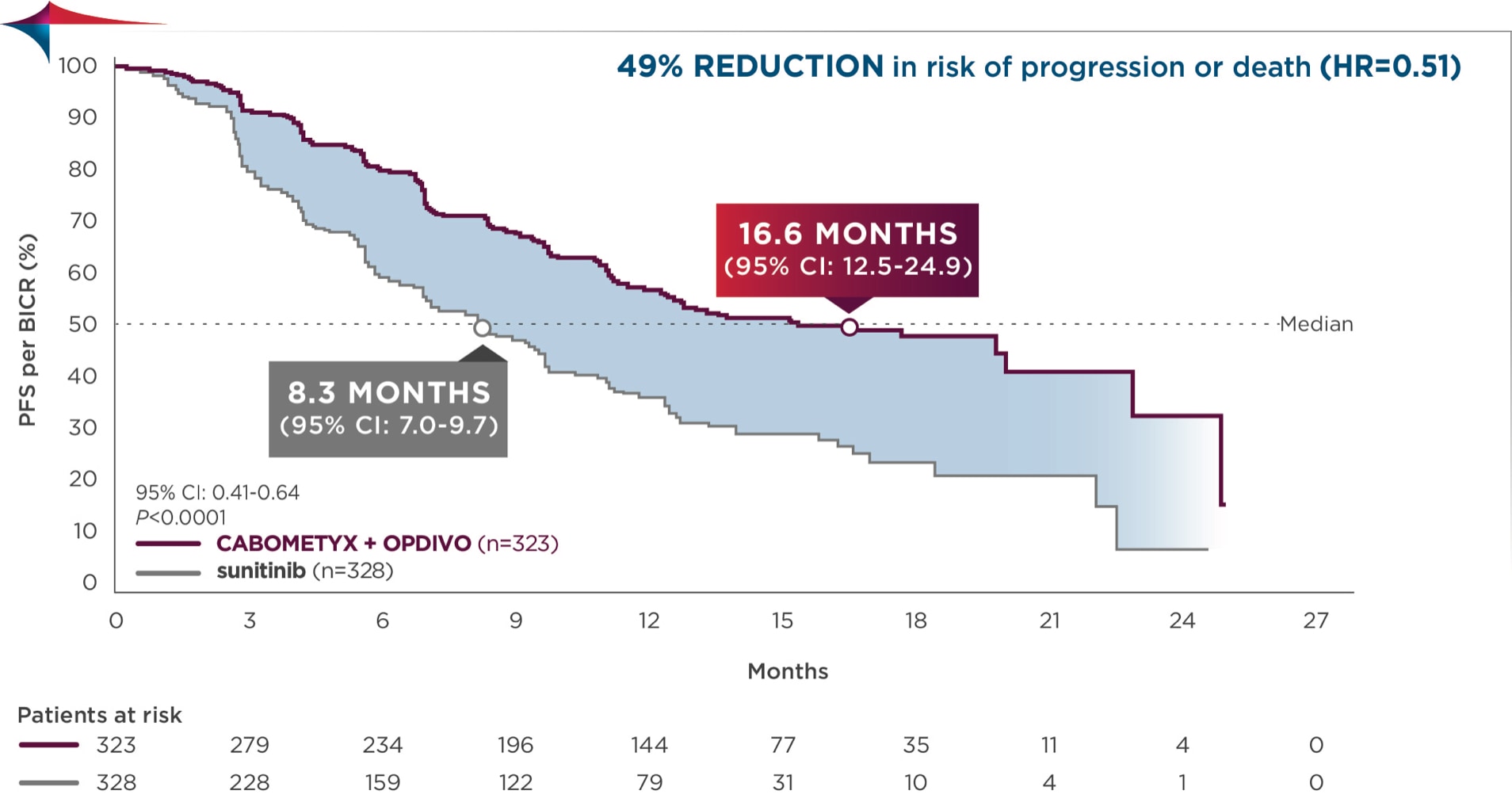
Median follow-up time of 18.1 months; range: 10.6-30.6 months2
PFS was assessed by BICR4
Consistent results for PFS were observed across the prespecified subgroup of IMDC risk categories4
5-YEAR MINIMUM FOLLOW-UP ANALYSIS
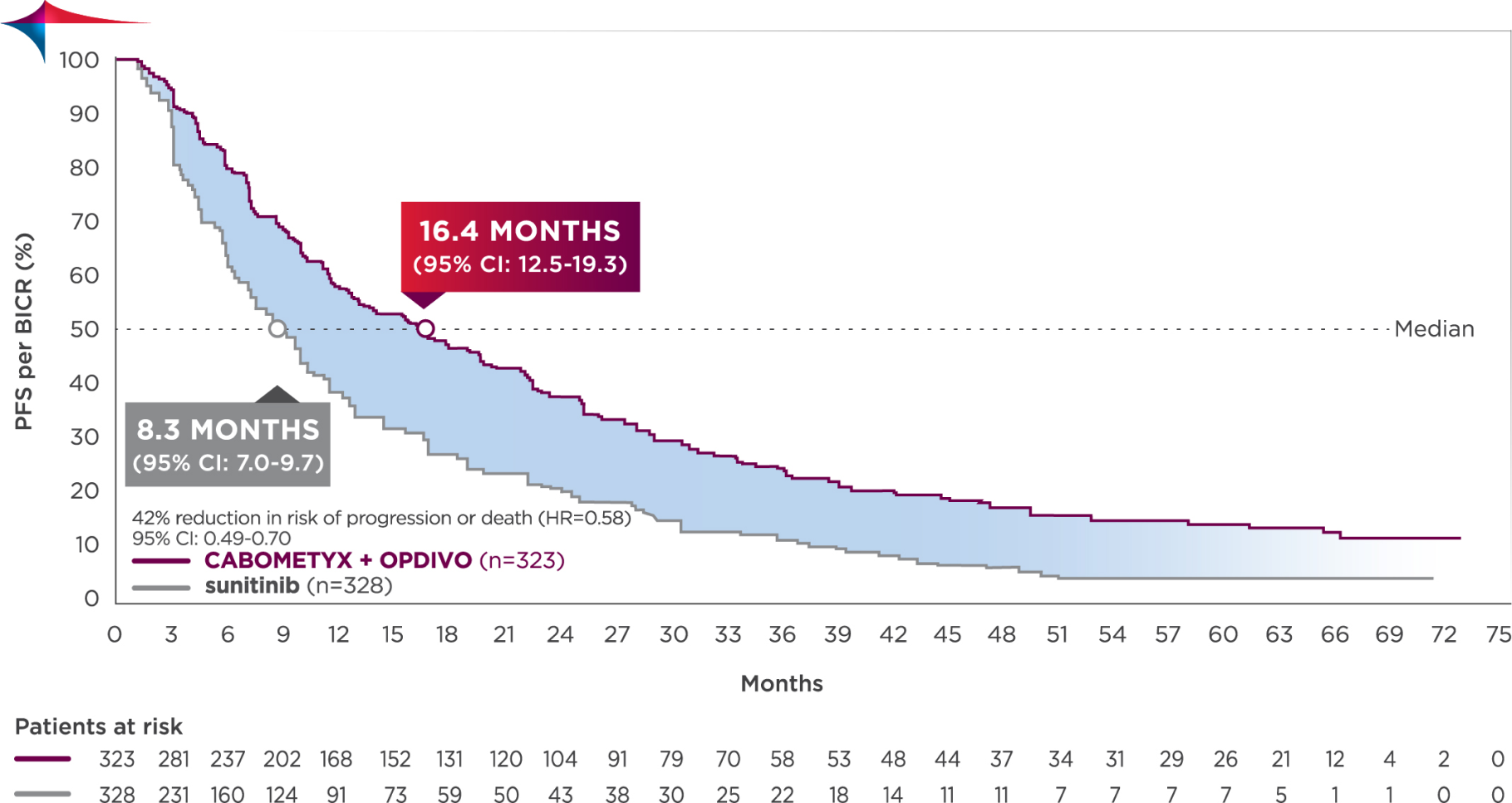
Median follow-up time of 67.6 months; range: 60.2-80.2 months3
Longest available follow-up for CheckMate-9ER
Median PFS was 16.4 months with CABOMETYX + OPDIVO vs 8.3 months with sunitinib3
No formal statistical testing was conducted at the time of the updated analysis.
Overall survival (OS), secondary endpoint4,6
Primary analysis
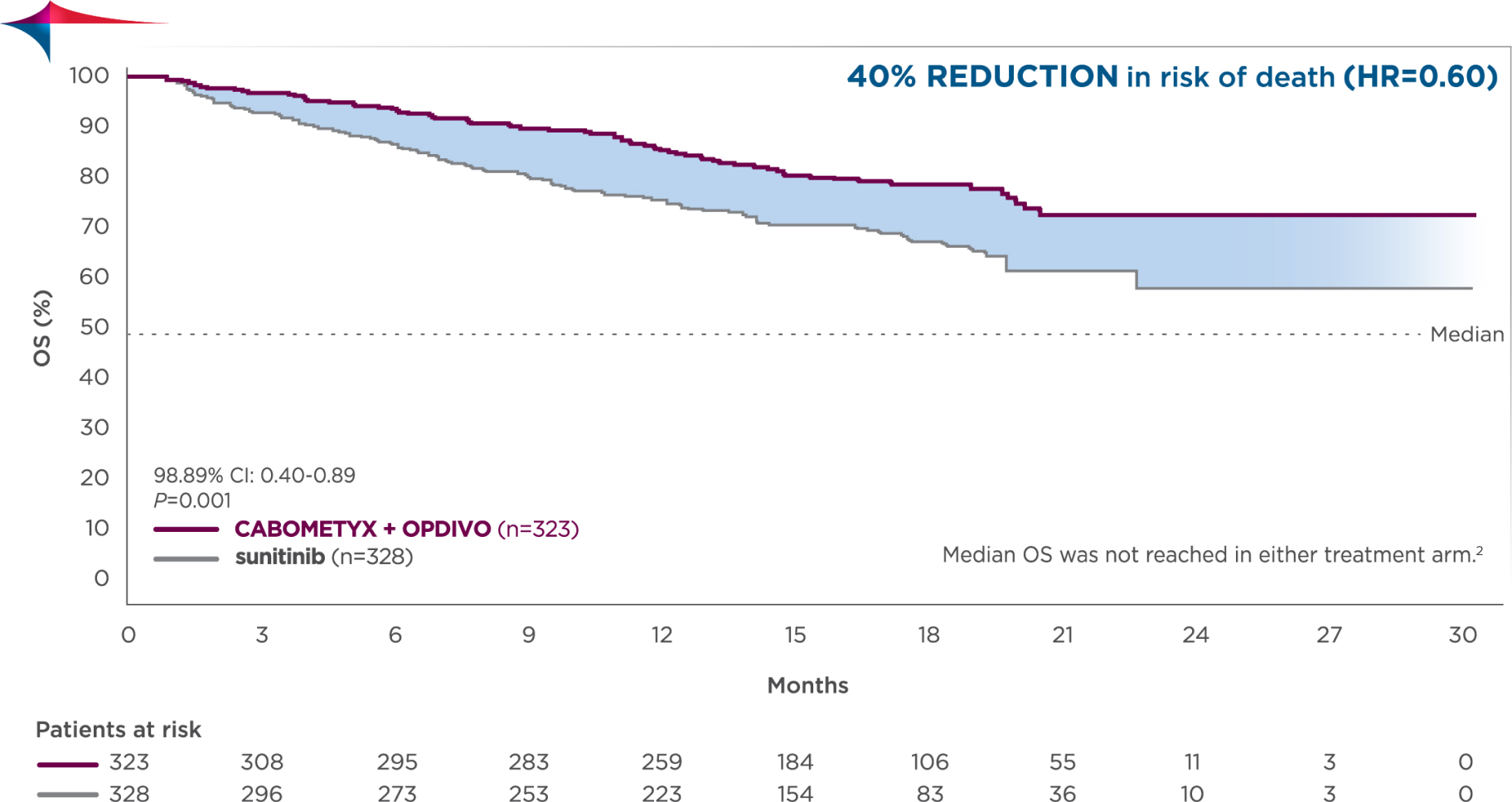
Median follow-up time of 18.1 months; range: 10.6-30.6 months2
- Pre-planned final analysis of OS (median follow-up: 32.9 months; range: 25.4-45.4 months): Median OS was 37.7 months for CABOMETYX + OPDIVO (95% Cl: 35.5-NR; n=323) compared with 34.3 months for sunitinib (95% Cl: 29.0-NR; n=328); HR=0.70 (95% Cl: 0.55-0.90)4,7,8
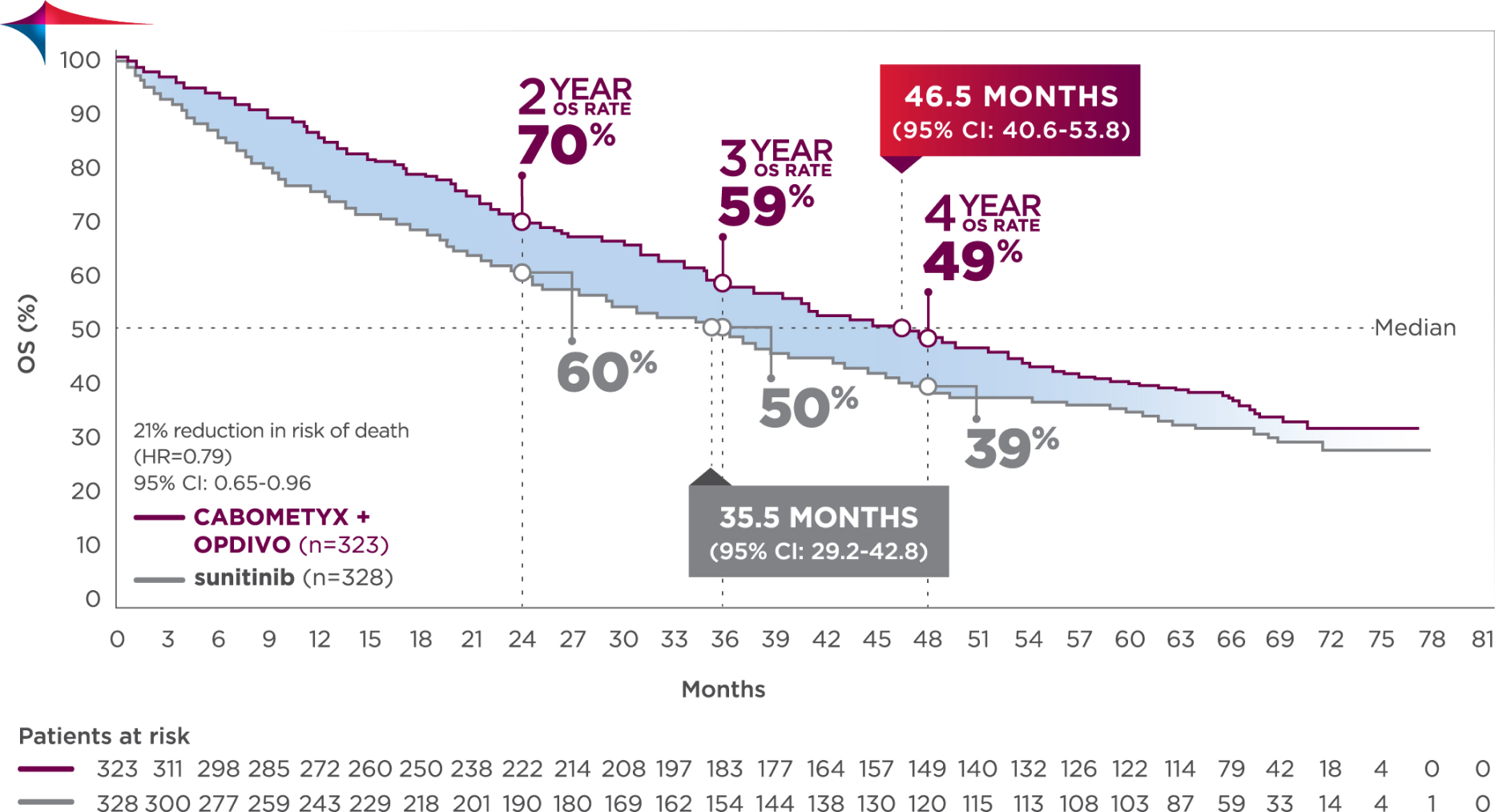
Median follow-up time of 67.6 months; range: 60.2-80.2 months3
Longest available follow-up for CheckMate-9ER
CABOMETYX + OPDIVO had a median OS of 46.5 months vs 35.5 months with sunitinib3
No formal statistical testing was conducted at the time of the updated analysis.
Objective response rate (ORR), secondary endpoint4,6
Primary analysis in the ITT population4
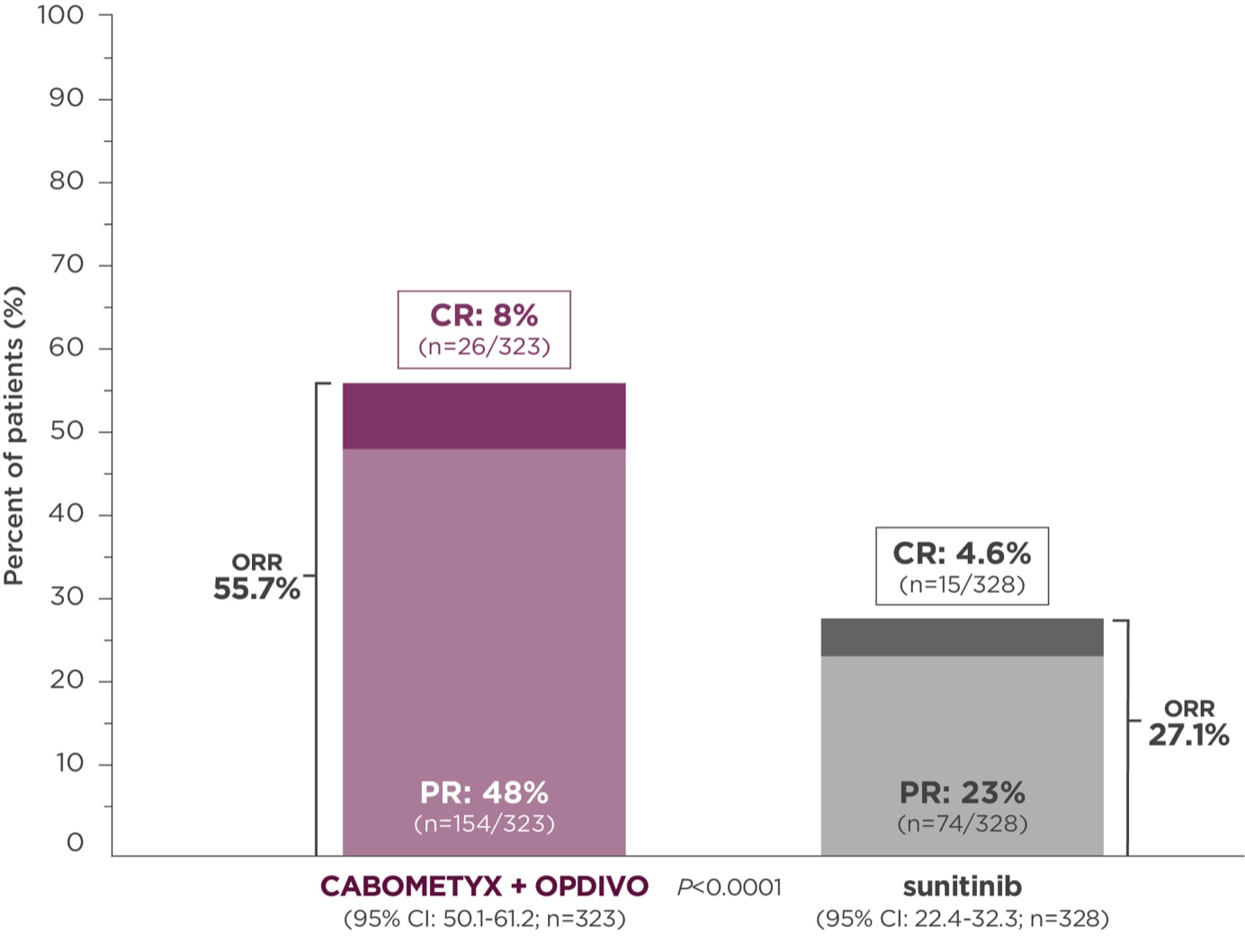
Median follow-up time of 18.1 months; range: 10.6-30.6 months2
ORR was assessed by BICR4
At the time of primary analysis, only 5.6% of patients had progressive disease with CABOMETYX + OPDIVO vs 13.7% of patients with sunitinib2#
#Progressive disease: At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. (Note: The appearance of 1 or more new lesions is also considered progression.)5
5-YEAR minimum follow-up analysis
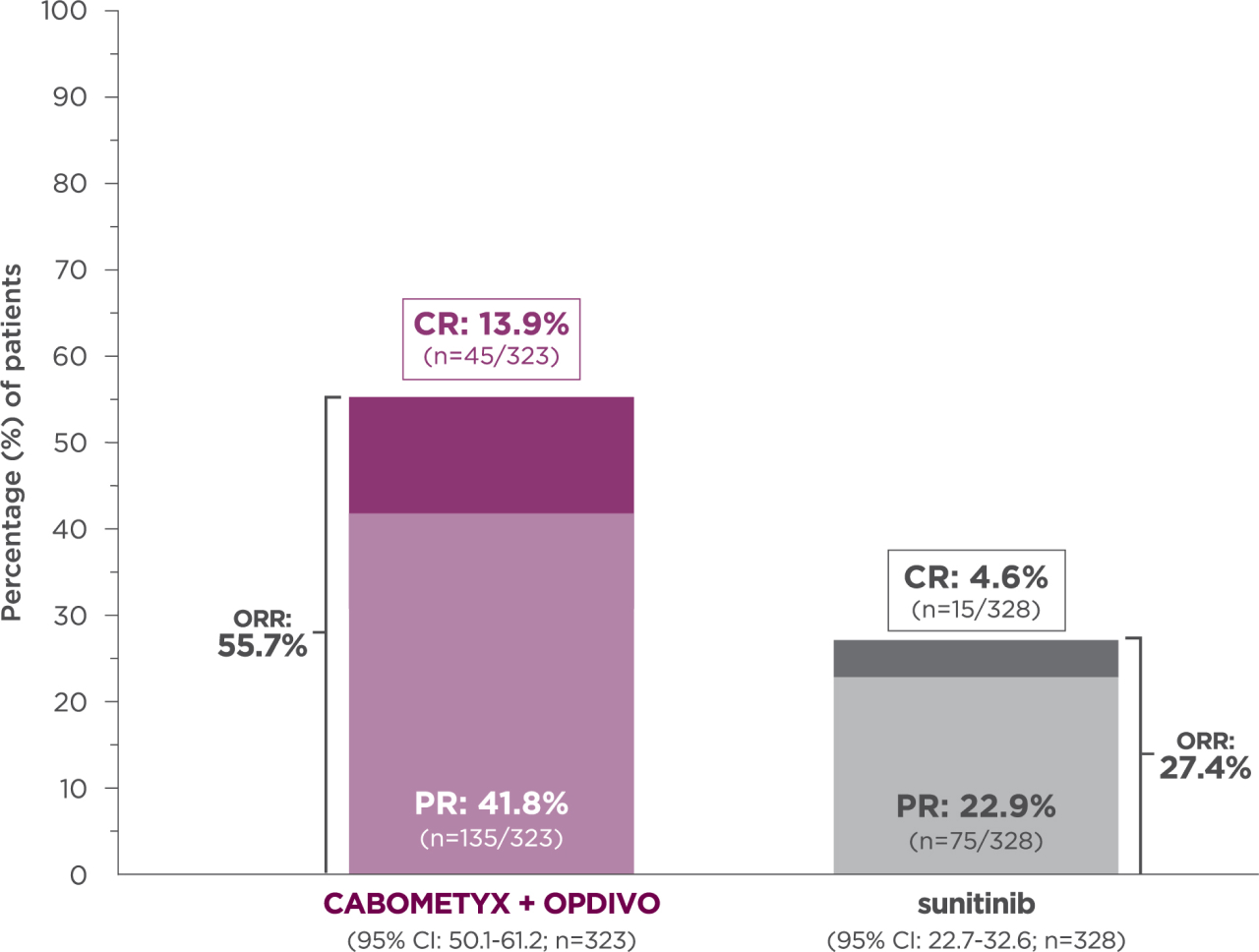
Median follow-up time of 67.6 months; range: 60.2-80.2 months3
Longest available follow-up for CheckMate-9ER
13.9% of patients achieved a CR with CABOMETYX + OPDIVO vs 4.6% of patients with sunitinib3
No formal statistical testing was conducted at the time of the updated analysis.
Select Important Safety Information
The full Prescribing Information for CABOMETYX includes Warnings and Precautions for: hemorrhage, perforations and fistulas, thrombotic events, hypertension and hypertensive crises, diarrhea, palmar-plantar erythrodysesthesia (PPE), hepatotoxicity, adrenal insufficiency, proteinuria, osteonecrosis of the jaw, impaired wound healing, reversible posterior leukoencephalopathy syndrome, thyroid dysfunction, hypocalcemia, and embryo-fetal toxicity.
See additional Important Safety Information below.
national comprehensive cancer network® (NCCN®)
PREFERRED OPTION10
Cabozantinib (CABOMETYX) + nivolumab (OPDIVO) was the first TKI + IO regimen with an NCCN recommendation in both clear-cell and non-clear-cell aRCC10

- Category 1, preferred option across all risk groups in 1L clear-cell RCC10
- NCCN Category 1: Based upon high-level evidence (≥1 randomized phase 3 trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate10

- Category 2A, preferred option in non-clear-cell RCC10
- NCCN Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate10
-
NCCN makes no representations or warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. Recommendations made by NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Kidney Cancer, V.3.2025.10
-
The approval of CABOMETYX with OPDIVO in 1L aRCC was based on a randomized (1:1), open-label, Phase 3 trial of CABOMETYX + OPDIVO vs sunitinib in patients with previously untreated aRCC with a clear-cell component.
1L=first-line; aRCC=advanced renal cell carcinoma; BICR=blinded independent central review; CI=confidence interval; CR=complete response; HR=hazard ratio; HRQoL=health-related quality of life; IMDC=International Metastatic RCC Database Consortium; IO=immunotherapy; ITT=intent to treat; IV=intravenous; NR=not reached; ORR=objective response rate; OS=overall survival; PD-L1=programmed cell death ligand 1; PFS=progression-free survival; PFS-2=progression-free survival after subsequent therapy; PK=pharmacokinetics; PO=by mouth; PR=partial response; RECIST=Response Evaluation Criteria in Solid Tumors; TKI=tyrosine kinase inhibitor.
References:
- Data on file. IQVIA National Prescription Audit, December 2024. Exelixis, Inc.
- Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841.
- Data on file. Exelixis, Inc.
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma [protocol]. N Engl J Med. 2021;384(9):829-841.
- Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal cell carcinoma: outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. Poster presented at Genitourinary Cancers Symposium; February 11-13, 2021.
- Motzer RJ, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomized, phase 3 trial. Lancet Oncol. 2022;23(7):888-898.
- Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with 2-year follow-up in CheckMate 9ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. Poster presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium; February 17-19, 2022.
- OPDIVO® (nivolumab) Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Kidney Cancer V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed January 9, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.

