Previously treated epNET efficacy data
CABOMETYX doubled median PFS in epNET1
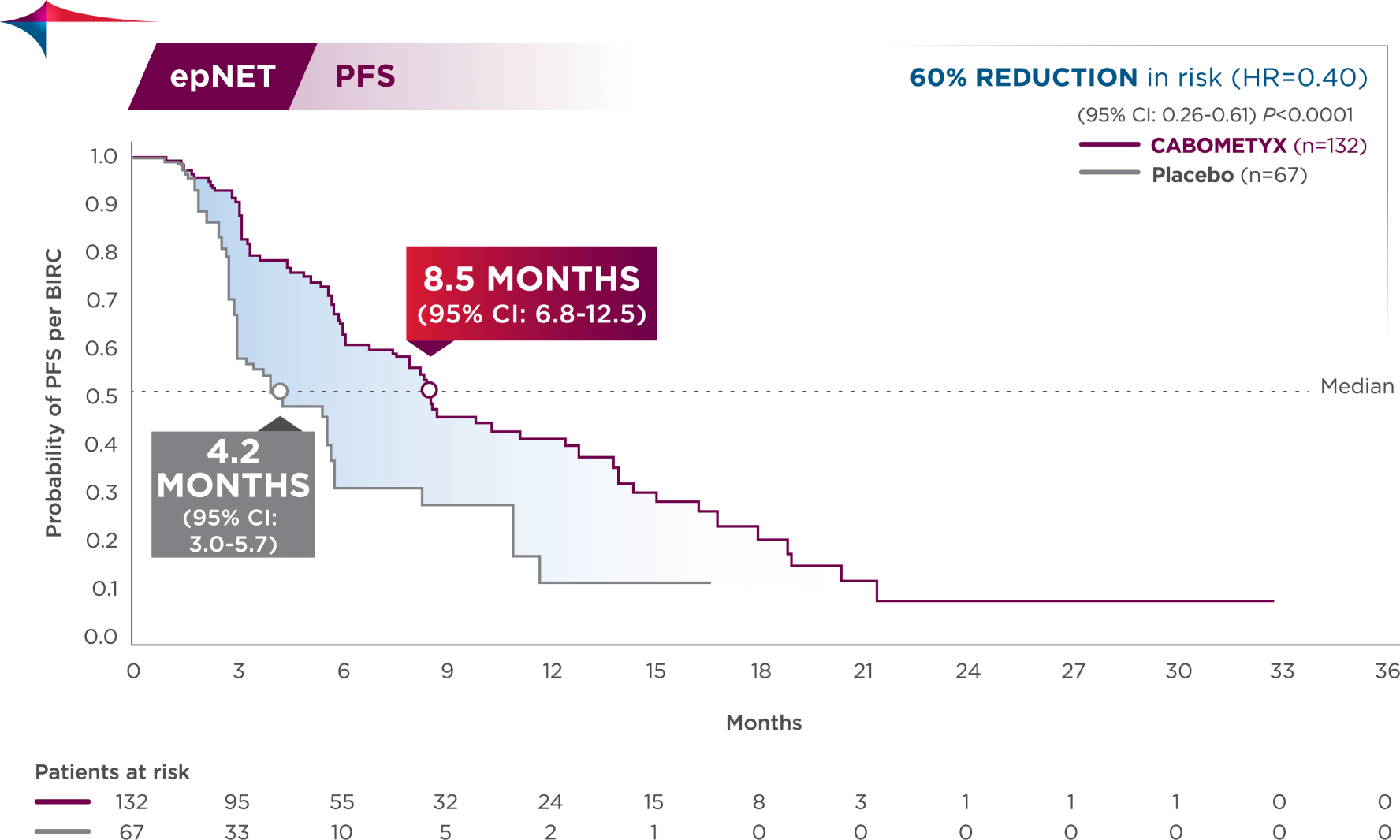
60% reduction in risk of progression or death
PFS results in epNET patients across select disease characteristics2
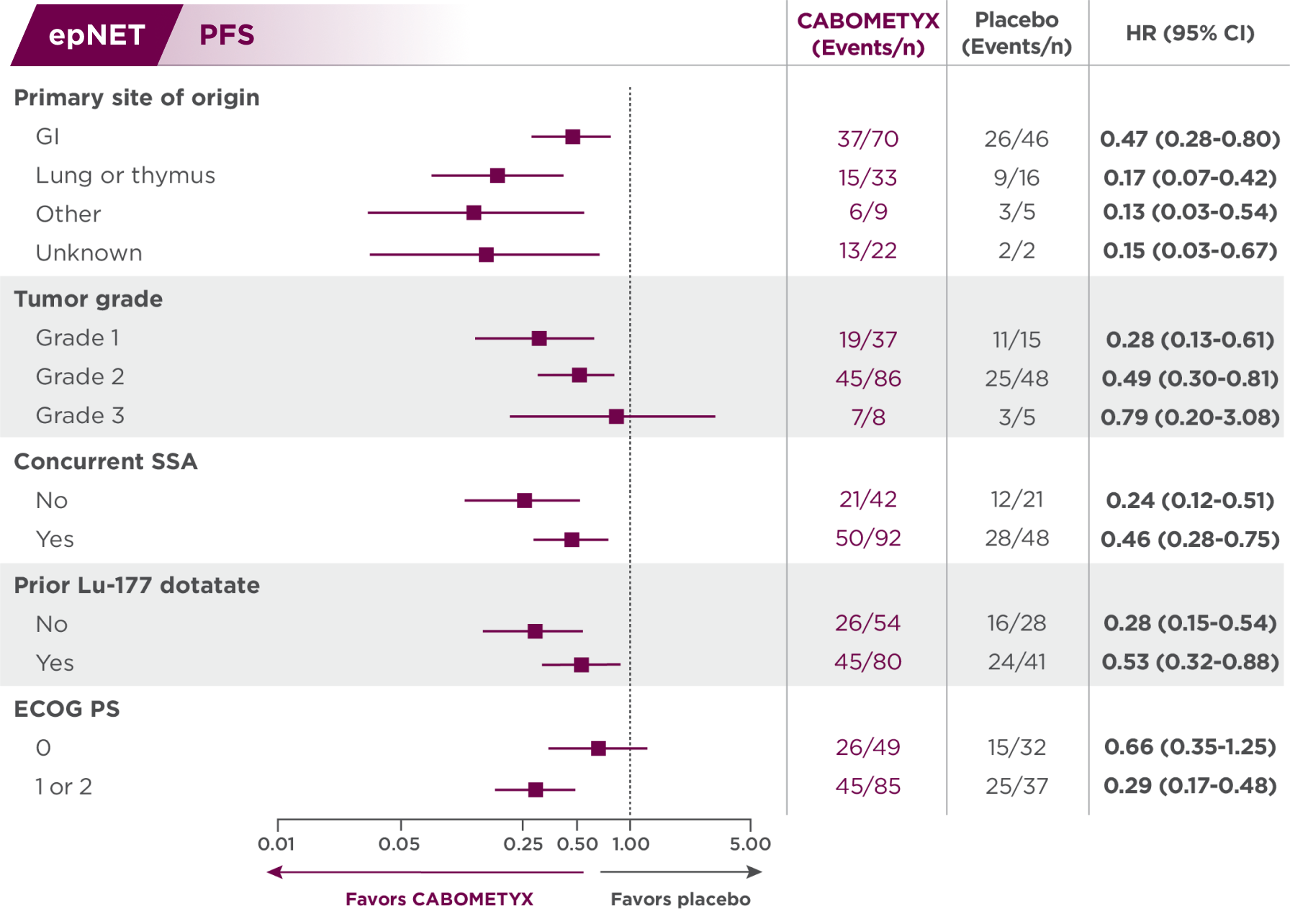
Subgroups were not powered to show differences between treatment arms, and results should be considered hypothesis generating.
Descriptive analysis
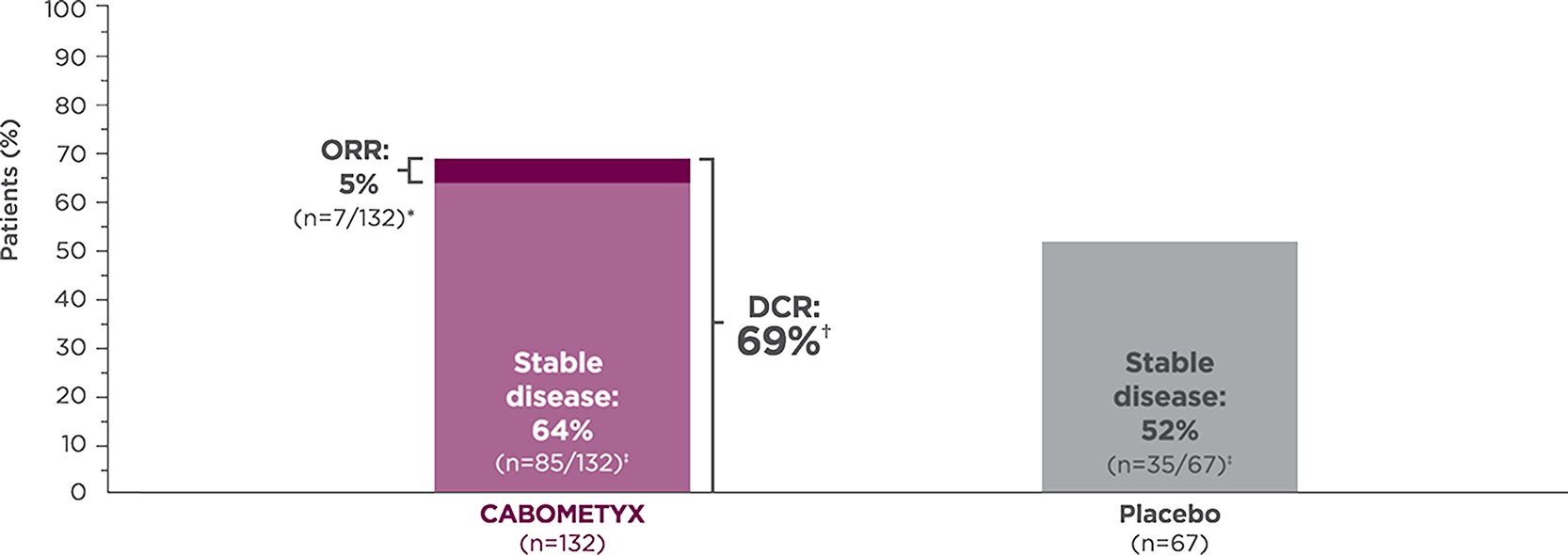
Tumor response with CABOMETYX in epNET1,3
epNET
CABOMETYX achieved 69% disease control
- *
-
All responses confirmed were partial responses.2
- †
-
Disease control rate is defined as the percentage of patients with a complete response, partial response, or stable disease, as measured by RECIST v1.1.3
- ‡
-
Stable disease is defined as neither sufficient shrinkage to qualify as partial response nor sufficient increase to qualify as PD.4 Stable disease may reflect the natural history of disease rather than any effect of the drug.
Descriptive analysis
Reduction in target lesions from baseline2,5
Of the 203 epNET ITT patients, 174 were evaluable (n=111 in the CABOMETYX arm, n=63 in the placebo arm).

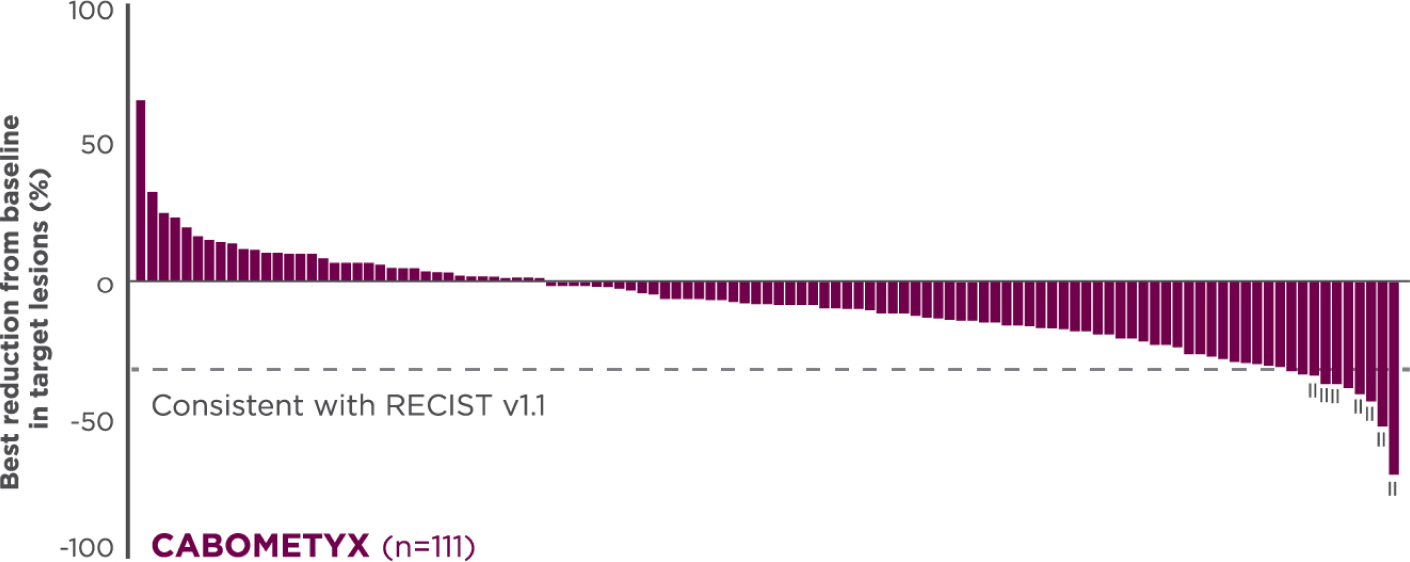
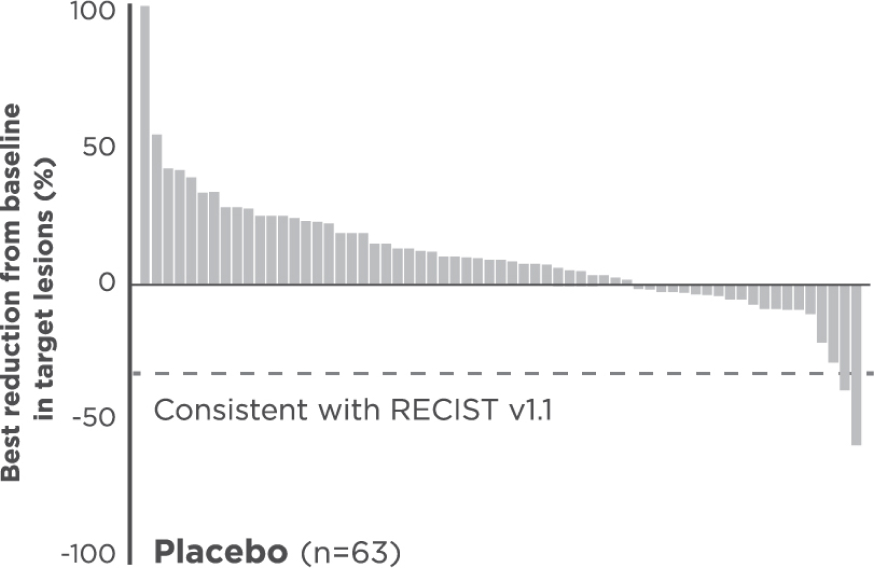
Interpret data with caution.
No formal statistical testing was conducted for the tumor assessment waterfall plots.
Best reduction is maximum reduction in sum of diameters of target lesions. Patients with target lesion at baseline and at least 1 on-treatment tumor assessment are included. Some patients are not represented due to lack of evaluable postbaseline assessment, censoring before first evaluable postbaseline assessment, lack of target lesions, and/or incomplete or inevaluable target-lesion assessment. Data from time points after the first date of any of the censoring events defined for the primary PFS analysis were excluded.3
- §
-
Each vertical line represents 1 patient.5
- II
-
Indicates confirmed partial responses per BIRC.5
Updated analysis
OS results from CABINET1
OS data were not mature at the time of the updated analysis and may be impacted by crossover
epNET
Updated OS
63% | Deaths | 60% | Deaths | (HR=1.05; 95% CI: 0.71-1.54) | |
37% of placebo patients crossed over to open-label CABOMETYX | |||||
63% | Deaths |
60% | Deaths |
(HR=1.05; 95% CI: 0.71-1.54) | |
37% of placebo patients crossed over to open-label CABOMETYX | |
The CABINET trial was unblinded early and patients were allowed to cross over to open-label CABOMETYX regardless of whether they had experienced progression. A later updated OS analysis was conducted when 123 deaths were observed in the epNET cohort.
BIRC=blinded independent review committee; CI=confidence interval; DCR=disease control rate; ECOG PS=Eastern Cooperative Oncology Group performance status; epNET=extrapancreatic neuroendocrine tumors; HR=hazard ratio; ITT=intent to treat; Lu-177=lutetium-177; NET=neuroendocrine tumors; ORR=overall response rate; OS=overall survival; PD=progressive disease; PFS=progression-free survival; pNET=pancreatic neuroendocrine tumors; RECIST=Response Evaluation Criteria in Solid Tumors; SSA=somatostatin analogue.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- Chan JA, Geyer S, Zemla T, et al. Phase 3 trial of cabozantinib in previously treated advanced neuroendocrine tumors. N Engl J Med. 2024; Published online September 16, 2024. doi:10.1056/NEJMoa2403991.
- Data on file. Exelixis, Inc.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
- Chan JA, Geyer S, Zemla T, et al. Phase 3 trial of cabozantinib in previously treated advanced neuroendocrine tumors [supplementary appendix]. N Engl J Med. 2024; Published online September 16, 2024. doi:10.1056/NEJMoa2403991.


