For the first time, a Phase 3 trial encompasses the wide-ranging heterogeneity of NET1-6
On This Page:
CABINET: A randomized (2:1), double-blind, placebo-controlled, NCI-sponsored Phase 3 trial1,6
Patients with neuroendocrine tumors (NET) (N=298)1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| |||||||||||
Disease progression | Disease progression | Disease progression | Disease progression | ||||||||
| |||||||||||
- Primary endpoint: PFS by BIRC1
- Secondary endpoints: ORR, OS, safety, and tolerability6†
- *
-
Unblinding and crossover to open-label CABOMETYX allowed after confirmation of progressive disease by real-time central radiology review.1
- †
-
As recommended by the Data and Safety Monitoring Board, the CABINET trial was unblinded before the final prespecified efficacy analysis, allowing all remaining placebo patients to cross over to CABOMETYX.1
Inclusion criteria6:
- Progression or intolerance following ≥1 FDA-approved systemic therapy, not including SSAs
- Well- to moderately-differentiated NET
- Functional and nonfunctional NET
- Tumor Grades 1-3
- ECOG PS 0-2
- Disease progression ≤12 months before randomization
- Concurrent SSA use permitted if a stable dose was received for ≥2 months
CABINET allowed all sites of origin, including the lungs, GI tract, and pancreas6
-
CABINET was sponsored by the National Cancer Institute (NCI), a part of the National Institutes of Health, and initiated in 2018 by the NCI-funded National Clinical Trials Network group, the Alliance for Clinical Trials in Oncology (Alliance), to address the unmet needs in NET.6
-
Tumor assessments were done every 12 weeks by radiographic imaging for tumor response and progression (as determined by RECIST v1.1).6
CABINET enrolled a broad patient population, allowing patients across all sites of origin6
CABINET patients by site of origin7
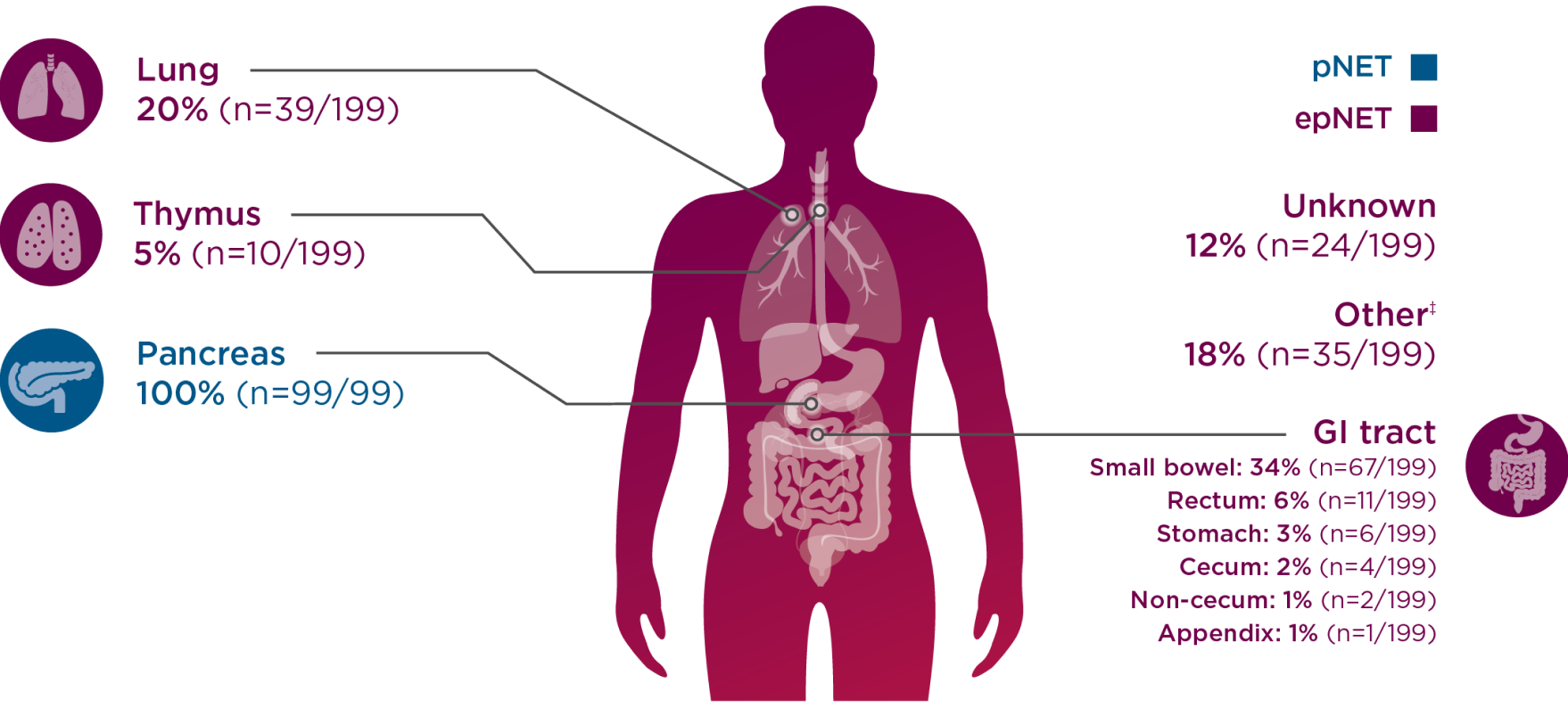
-
Percentages for the epNET cohort exceed 100 due to rounding.
-
‡Other sites included small bowel, mesentery, ampulla, midgut, hindgut, biliary tract, larynx, presacral space, kidney, and ethmoid sinus.7
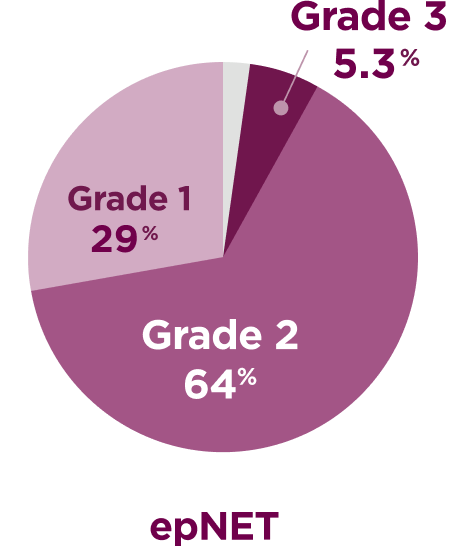
CABINET evaluated a range of patients, >70% with Grade 2-3 tumors6,7

Tumor grade
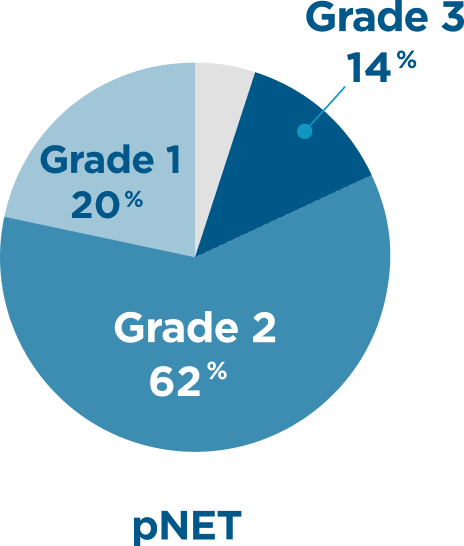
- Placebo arm tumor grade distribution for pNET: 21% Grade 1; 67% Grade 2; 9.1% Grade 3; 3% unknown
- Placebo arm tumor grade distribution for epNET: 22% Grade 1; 67% Grade 2; 7.5% Grade 3; 3% unknown
Tumor grade percentages do not total 100 due to rounding.
Lu-177 dotatate use
Patients who received prior Lu-177 dotatate
SSA use
Patients who received concurrent SSA
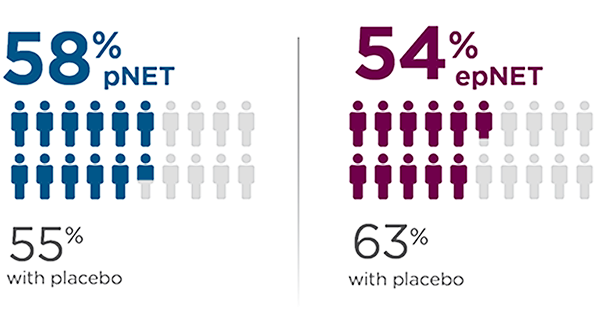
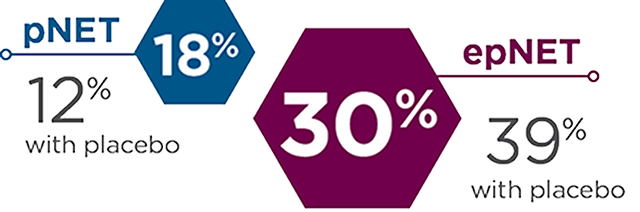
Prior SSA use
Patients who received prior SSA

Hormone syndrome
Patients with functional disease
Some patients had unknown functional status.
CABINET was the first Phase 3 trial across pNET and epNET to include patients with prior peptide receptor radionuclide therapy (PRRT)3,5,8-10
Select patient demographics7
pNET cohort (N=99) | epNET cohort (N=199) | |||
CABOMETYX | Placebo | CABOMETYX | Placebo | |
Median age in years (range) | 60 (29-78) | 64 (39-79) | 66 (28-86) | 66 (30-82) |
Gender, n (%) | ||||
Male/Female | 37 (56)/29 (44) | 19 (58)/14 (42) | 60 (45)/72 (55) | 37 (55)/30 (45) |
ECOG PS, n (%) | ||||
0/1/2 | 36 (55)/ | 18 (55)/ | 48 (36)/ | 29 (43)/ |
Sites of metastatic disease, n (%) | ||||
Liver | 65 (98) | 30 (91) | 117 (89) | 62 (93) |
Lymph nodes | 30 (45) | 18 (55) | 91 (69) | 49 (73) |
Bone | 19 (29) | 7 (21) | 67 (51) | 33 (49) |
Lungs | 4 (6) | 2 (6) | 29 (22) | 13 (19) |
Abdominal wall | 3 (4.5) | 1 (3) | 10 (8) | 7 (10) |
Subcutaneous tissue | NA | NA | 5 (3.8) | 1 (1.5) |
CNS/Brain | NA | NA | 4 (3) | 3 (4.5) |
Other§ | 10 (15) | 5 (15) | 47 (36) | 23 (33) |
BIRC=blinded independent review committee; CNS=central nervous system; ECOG PS=Eastern Cooperative Oncology Group performance status; GI=gastrointestinal; Lu-177=lutetium-177 NA=not available; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors; SSA=somatostatin analogue.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- AFINITOR® (everolimus) Prescribing Information. Novartis Pharmaceuticals Corporation.
- SUTENT® (sunitinib malate) Prescribing Information. Pfizer, Inc.
- LUTATHERA® (lutetium Lu-177 dotatate) Prescribing Information. Novartis Pharmaceuticals Corporation.
- SOMATULINE® DEPOT (lanreotide) Prescribing Information. Ipsen Pharma Biotech.
- Chan JA, Geyer S, Zemla T, et al. Phase 3 trial of cabozantinib in previously treated advanced neuroendocrine tumors. N Engl J Med. 2024; Published online September 16, 2024. doi:10.1056/NEJMoa2403991.
- Data on file. Exelixis, Inc.
- US Food and Drug Administration. FDA approves new treatment for certain digestive tract cancers. January 26, 2018. Accessed September 5, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-digestive-tract-cancers.
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514-523.
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968-977.



